Insights
Informing Food Policy Reform with Research and Analysis
The United States is facing a metabolic and chronic disease crisis. Today, more than 60 percent of adults have at least one chronic disease. Diet is a major contributing factor to several chronic diseases, including obesity, cardiovascular disease, hypertension, stroke, type 2 diabetes, and some cancers.
Promoting consumers’ access to nutritious, affordable food and encouraging consumers to adopt healthy eating habits—such as avoiding ultra-processed foods and eating more fruits, vegetables, and whole grains—may help to decrease chronic disease in the United States. Additionally, reformulation of food products by manufacturers would help to make some processed foods healthier.
The new Administration and Congress alike are consequently focused on these issues.
Research to Support Food Policy Reform Initiatives
RTI International is a leader in providing rigorous research and policy analysis to support federal agencies and policymakers on making the types of policy changes that are being considered. Research-based policy changes are likely to improve buy-in, increase impact, and reduce unintended consequences.
RTI experts have a long history of conducting studies to help inform the U.S. Food and Drug Administration’s (FDA’s) rulemaking process. For example, when the FDA was considering regulation of trans fat, RTI supported the FDA by assessing the costs and benefits of requiring changes to the Nutrition Facts Label to add information on trans fats. As part of this study, our researchers estimated the number of products that would be reformulated rather than relabeled and predicted changes in American diets because of regulatory changes. The FDA used the results of RTI’s study to help inform a new regulation requiring manufacturers to list trans fats on the Nutrition Facts Label.
Our Experts
RTI has also developed several tools that the FDA can use to inform the rulemaking process for the Substances Generally Recognized as Safe (GRAS) Final Rule and other regulatory changes to food policy, including
- the FDA Labeling Cost Model to estimate the cost to industry of a new labeling requirement, and
- the FDA Reformulation Cost Model to estimate the cost to industry of reformulating food products in response to regulation.
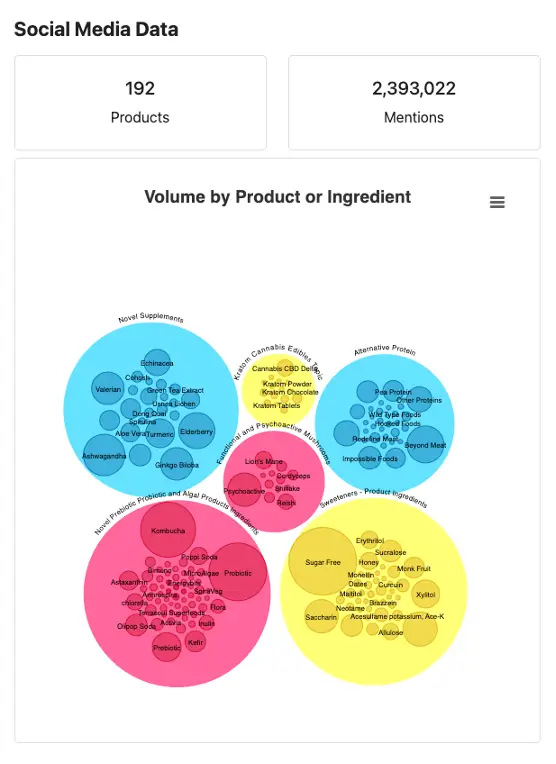
Recently, RTI developed a dashboard with data visualization capabilities to monitor novel foods, including alternative protein sources, sweeteners and other additives, and probiotic and algal products. This dashboard triangulated FDA adverse events data, social media listening data, and Google Trends data to monitor the novel food landscape. The FDA can use the tool to help detect potential signals of adverse events earlier. This dashboard could be leveraged to aid the FDA in rulemaking related to eliminating the option for food companies to designate a substance as GRAS without FDA review.
The Novel Foods Dashboard that RTI developed for the FDA uses data visualization to summarize social media listening data on consumer discourse about novel food products.
RTI also has experience conducting consumer research to inform rulemaking regarding product labeling. In a recent study for the U.S. Department of Agriculture’s (USDA’s) Food Safety and Inspection Service, RTI conducted an experimental study to measure consumers’ awareness of, knowledge of, and willingness to pay for “Product of USA” labeling claims. Based on the results of this study, USDA issued a proposed rule in 2023 that allows the voluntary “Product of USA” or “Made in the USA” label claim to be used on meat, poultry, and egg products only when they are derived from animals born, raised, slaughtered, and processed in the United States.
Food Labeling and Messaging
RTI’s communication scientists understand that consumers may find it challenging to identify healthy foods and can be confused by manufacturer claims about processed food products. In a recent study, our researchers found that participants perceived snack foods with vitamin fortification claims as healthier than those without such claims. Additionally, they were more likely to choose the less healthy product with a claim over the healthier product without one, suggesting that these claims may be misleading.
If the FDA proposes labeling changes to help U.S. consumers make more informed food choices, it will be important to conduct consumer research to inform these changes. RTI has conducted formative research for the FDA to explore consumer response to allergen labeling, gluten-free labeling, and plant-based beverages.
In a recent study for the Robert Wood Johnson Foundation, RTI researchers explored the combined impact of placing a nutrition label on the front of soda highlighting sugar content and imposing a tax to reduce soda purchases by parents for their young children. Study results indicated that the combination of a front-of-pack nutrition label and a tax reduced the likelihood of a parent choosing a soda for their child more than either policy alone.
Looking Ahead to Food Policy Impact and Communication
Any future policy changes being considered will require research on the impacts and clear communication to consumers and other stakeholders about the changes. The following are just some examples of the types of research that RTI could conduct to support policy changes:
- Literature reviews on specific food ingredients or ultra-processed foods to summarize what is already known and where gaps remain to highlight priorities for new research.
- Formative research with food industry executives, growers and producers, and other stakeholders to identify facilitators and barriers to the potential policy changes.
- Message testing with consumers to assess consumer understanding of potential labeling changes on health beliefs and intention to purchase.
- Message testing with health care providers—including nutritionists—to ensure that they have the resources they need to share new dietary guidelines with patients in a way that makes them more likely to be implemented as intended.
- Monitoring food industry claims in direct-to-consumer marketing content to help ensure compliance with any new FDA policies.
Food policy changes that are informed by evidence and evaluated throughout the regulatory process are likely to result in the most impactful changes to health and chronic disease rates. However, more funding is necessary to conduct this critical research. In the past, research funding on food products has been limited compared with other FDA-regulated products, setting back essential research and discoveries.
Learn more about RTI’s food and nutrition policy research capabilities.
Let’s work together to research the impact of food policy changes on Americans’ health and well-being
Disclaimer: This piece was written by Sheryl C. Cates (Senior Research Scientist), Molly M. Lynch (Director, Audience Engagement Research Program), and Bridget Kelly (Health Communication Research Scientist) to share perspectives on a topic of interest. Expression of opinions within are those of the author or authors.